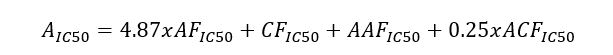Background: This article is a continuation of Theoretical Analysis for the Safe Form and Dosage of Amygdalin Product and Theoretical Study of the Process of Passage of Glycoside Amides through the Cell Membrane of Cancer Cell. They consider some possible natural modifications and hypothesize that it is not nitrile glycosides that have antitumor properties but their amide / carboxyl derivatives. The possibility of using this circumstance in conservative oncology is also considered. A mechanism for crossing the cell membrane and overcoming the immune functions of the cancer cell is presented.
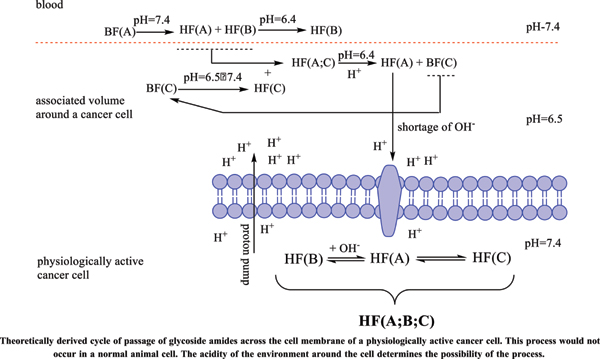
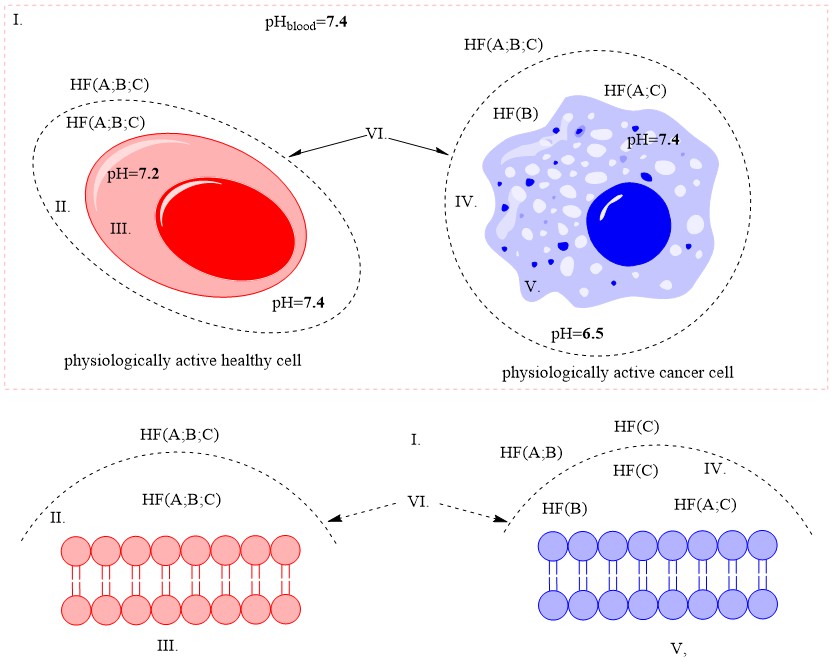
The physiologically active cancer cell itself is quite inert to external influences. It is far more stable than any physiologically active structural and/or functional organismal cell. Its defenses are discussed in detail in the article, and its main weakness was defined, namely: the cancer cell feeds mainly on carbohydrates and/ or carbohydrate complexes. In an effort to preserve its gene set, it has evolved to counteract biologically active substances by maximally preventing its passage through its cell membrane.
It is this property that could be used to minimize its effect on the whole body. In the same article, based on theoretical calculations and literature references, a hypothesis is stated: cancers could turn from severe infectious to controlled chronic ones (similar to diabetes, chronic hepatitis, etc.)
Objective: The pharmaceutical form allows deviation from the chemically pure substance. It is a convenient and at the same time accessible (from a financial and/or technological point of view) form for admission by patients.
Due to the great variety of natural glycosamide nitriles (starting material for the production of amide/ carboxylic acid), modern pharmacology allows their combined intake by chemical nature and concentration of the active form crossing the cell membrane.
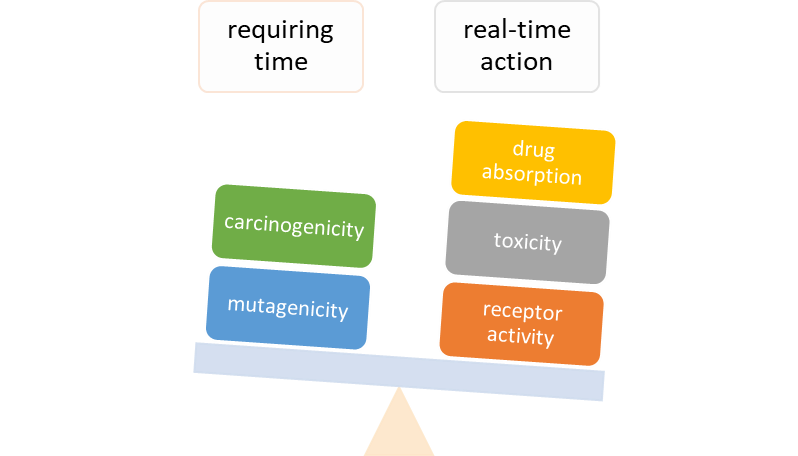
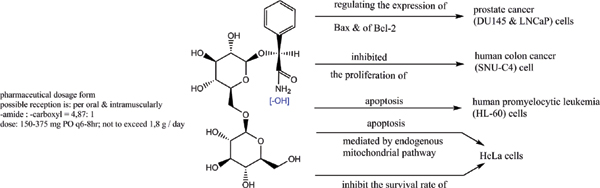
Natural nitrile glycosides hydrolyzed to amide/carboxylic acid are still unexplored but with great theoretical potential. As biologically active substances, these compounds also have significant toxicity. One of the purposes of this article is to organize laboratory tests on animals.
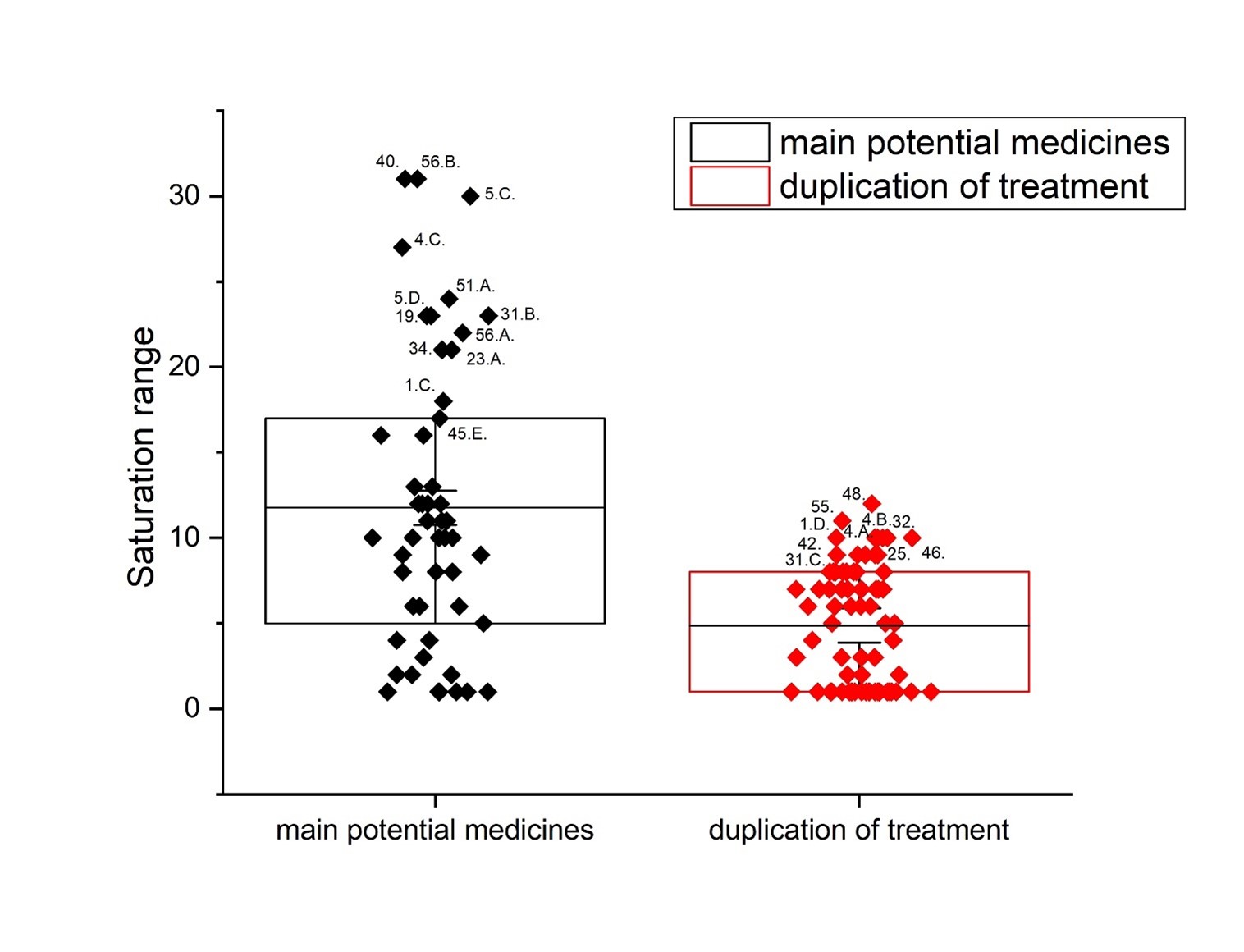
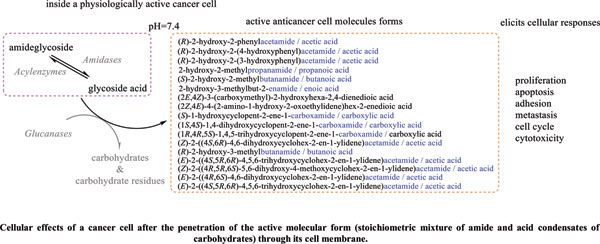
Methods: A comparative analysis is performed on the basis of stoichiometric calculations for the concentration of the active form and the prediction of the bioactivity. For this purpose, the following methodology is applied: Data analysis for active anticancer cell molecular form and Determination of the drug dose. The derived chemicals obtained immediately after the passage of glycosamide across the cancer cell membrane are: (R)-2-hydroxy-2-phenylacetamide, (R)-2- hydroxy-2-(4-hydroxyphenyl)acetamide, (R)-2-hydroxy-2-(3-hydroxyphenyl)acetamide, 2-hydroxy-2-methylpropanamide, (S)-2-hydroxy-2-methylbutanamide, 2-hydroxy-3-methylbut-2-enamide, (2Z,4E)-4-(2-amino-1-hydroxy-2-oxoethylide ne)hex-2-enedioic acid, (S)-1-hydroxycyclopent-2-ene-1-carboxamide, (1S,4S)-1,4-dihydroxycyclopent-2-ene-1-carbox amide, (1R,4R)-1,4,5-trihydroxycyclopent-2-ene-1-carboxamide, (Z)-2-((4S,6R)-4,6-dihydroxycyclohex-2-en-1-ylidene) acetamide, (R)-2-hydroxy-3-methylbutanamide, (E)-2-((4S,5R,6R)-4,5,6-trihydroxycyclohex-2-en-1-ylidene)acetamide, (Z)-2-((4R,5R,6S)-5,6-dihydroxy-4-methoxycyclohex-2-en-1-ylidene)acetamide, (E)-2-((4R,6S)-4,6-dihydroxycyclohex- 2-en-1-ylidene)acetamide and (E)-2-((4S,5R,6R)-4,5,6-trihydroxycyclohex-2-en-1-ylidene)acetamide.
Results: The use of two or more pharmaceutical forms would not prevent their penetration, subject to the mass ratios between the active antitumor amide and the active carboxyl transfer form.
Conclusion: Amides resulting from the hydrolysis of nitrile glycosides would have the ability to cross the cell membrane of a cancer cell and thus cause its cellular response. The pharmaceutical form must represent the exact amide / carboxylic acid ratio for the corresponding active anticancer cell form.
Tsanov, V., Tsanov, H. (2022) Theoretical Analysis of Anticancer Cellular Effects of Glycoside Amides, Anti-Cancer Agents in Medicinal Chemistry 2022; 22 (6), pp. 1171÷1200, Bentham Science Publisher
print ISSN: 1871-5206 | on-line ISSN: 1875-5992
DOI: 10.2174/1871520621666210903122831
HTML 5 version / 7MB single file size /
 Hello colleagues,
Hello colleagues,